Final Project
DNA Canvas
DNA Canvas is a biological platform for inexpensive patterning of bits and atoms at the nano scale.
Nanofabrication [1, 2] is the design and manufacture of structures in the nanometer range. Desigining and prototyping nanostructures is a prominent driver in a wide range of industries such as biomedicine, semiconductors, renewable energy, data storage and more. Deoxyribonucleic acid (DNA) as a molecular organizer was first suggested by Seeman in 1982 [3], and since materialized to be a promising bottom-up nanofabrication tool [4, 5]. DNA microscopy-by-sequencing [6, 7, 8, 9] is an emerging technique where spatial information is recovered at nanometric resolution by synthesized oligonucleotides (oligos) interactions. Current applications of DNA microscopy-by-sequencing in vivo include sub- cellular imaging and measurement of molecular interactions - far beyond optical limits. Here, we propose to develop DNA Canvas, a novel application of DNA microscopy-by-sequencing for nanofabrication.
We will fabricate a DNA Canvas by positioning uniquely barcoded oligos on a substrate, such that each oligo is immobilized and addressable. We will create a chip of area 50 μm^2 (approx. the diameter of a human hair) that contains as many as 25 million oligos (approx. human population in Shanghai). Each oligo has a unique barcode, and can be addressed by a complementary synthesized oligo attached with DNA, proteins, antibodies, monomers or other molecules of interest. Analogous to a screen, a DNA Canvas can “display” 25 MB images on a “screen” size of human hair (meaning, each pixel area is ∼10 nm^2 ). Similarly to a screen, the attached molecules (“pixels colors”) can be detached and the DNA Canvas can be re-used to “paint” a fresh image. Beyond artistic uses, the same technique can be applied for low-cost nanofabrication, where instead of “colors” - almost any protein/monomer/molecule can be accurately positioned on a 10 nm resolution. With exception of next-generation sequencing, which is currently offered as a service world-wide, the whole process of making and utilizing DNA Canvas doesn’t require any complicated or expensive machinery, outside of a standard BL1 lab. Furthermore, we will develop a protocol to replicate an existing DNA Canvas to identical copies in an inexpensive matter, further decreasing the amortized cost of the platform. Overall, DNA Canvas can enable nanofabrication to a wide range of students, startup companies and biohackers with limited means.
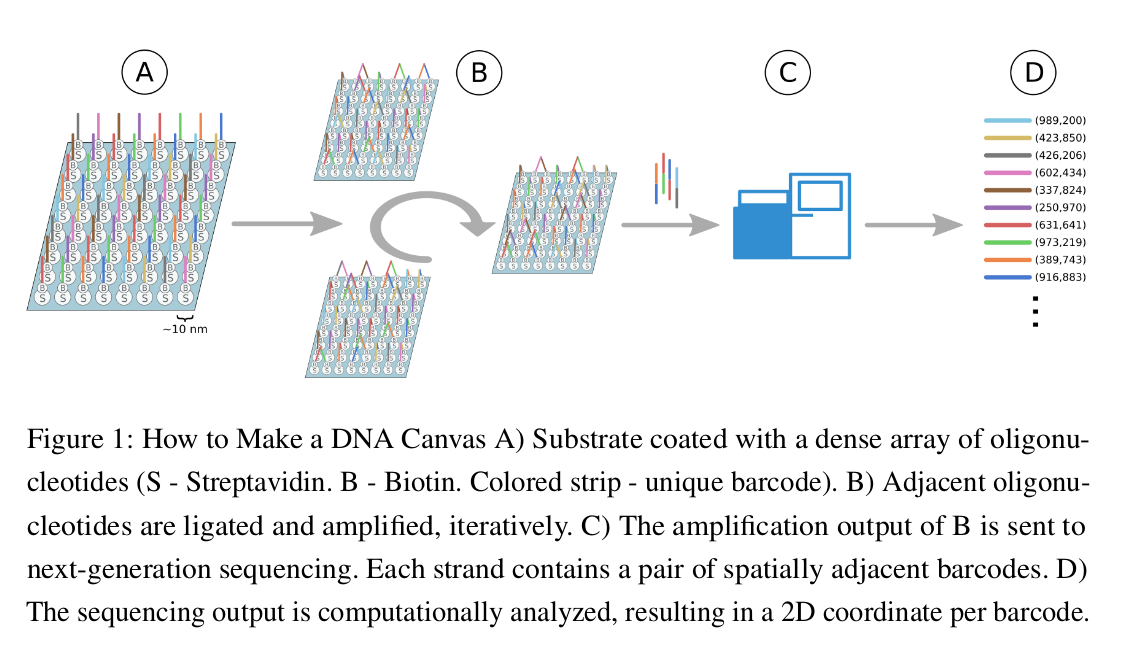
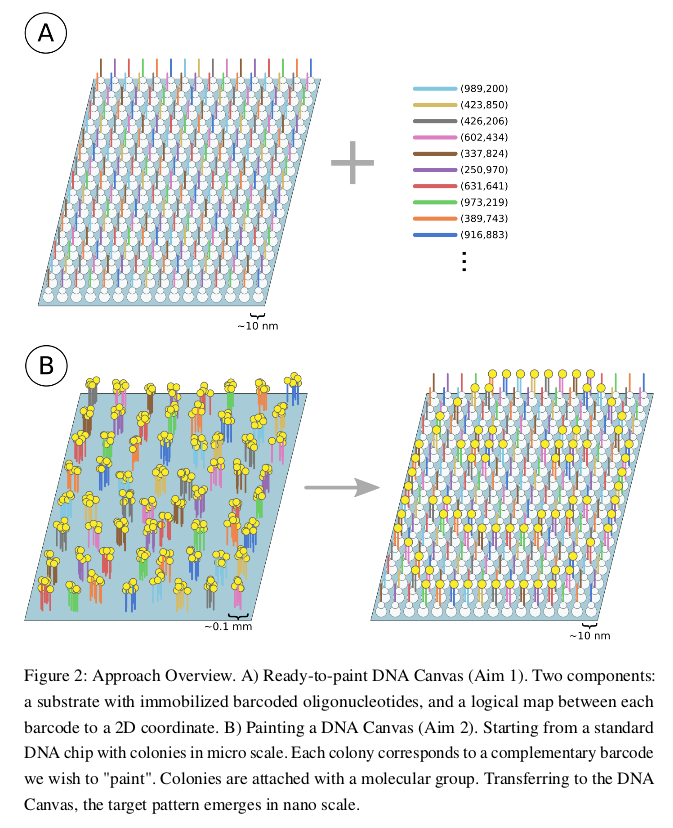
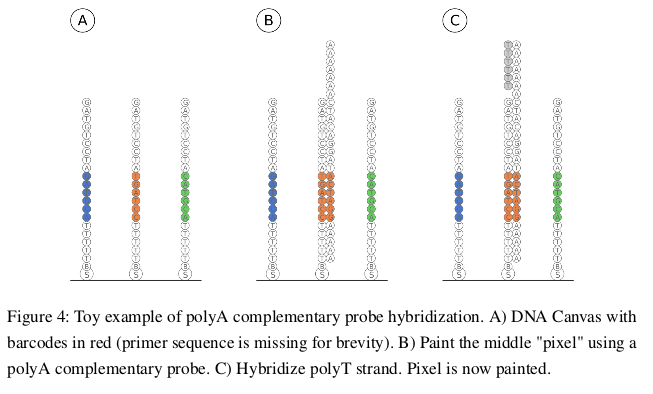
Video
A short animation made for the class to explain the basic process behind making DNA Canvas - immobilized oligos iterative ligation.
For more information, please contact eyalp@mit.edu
References:
[1] Ampere A Tseng. Nanofabrication: fundamentals and applications. World Scientific, 2008.
[2] Abhijit Biswas et al. “Advances in top–down and bottom–up surface nanofabrication: Techniques, applications & future prospects”. In: Advances in colloid and interface science 170.1-2 (2012), pp. 2–27.
[3] Nadrian C Seeman. “Nucleic acid junctions and lattices”. In: Journal of theoretical biology 99.2 (1982), pp. 237–247.
[4] Hanying Li, Joshua D Carter, and Thomas H LaBean. “Nanofabrication by DNA self-assembly”. In: Materials Today 12.5 (2009), pp. 24–32.
[5] Faisal A Aldaye, Alison L Palmer, and Hanadi F Sleiman. “Assembling materials with DNA as the guide”. In: science 321.5897 (2008), pp. 1795–1799.
[6] Alexander A Boulgakov, Andrew D Ellington, and Edward M Marcotte. “Bringing Microscopy-By-Sequencing into View”. In: Trends in biotechnology 38.2 (2020), pp. 154–162.
[7] Joshua A Weinstein, Aviv Regev, and Feng Zhang. “DNA microscopy: Optics-free spatio-genetic imaging by a stand-alone chemical reaction”. In: Cell 178.1 (2019), pp. 229–241.
[8] Alexander A Boulgakov et al. “From space to sequence and back again: iterative DNA proximity ligation and its applications to DNA-based imaging”. In: BioRxiv (2018), p. 470211.
[9] Thomas E Schaus et al. “A DNA nanoscope via auto-cycling proximity recording”. In: Nature communications 8.1 (2017), pp. 1–9